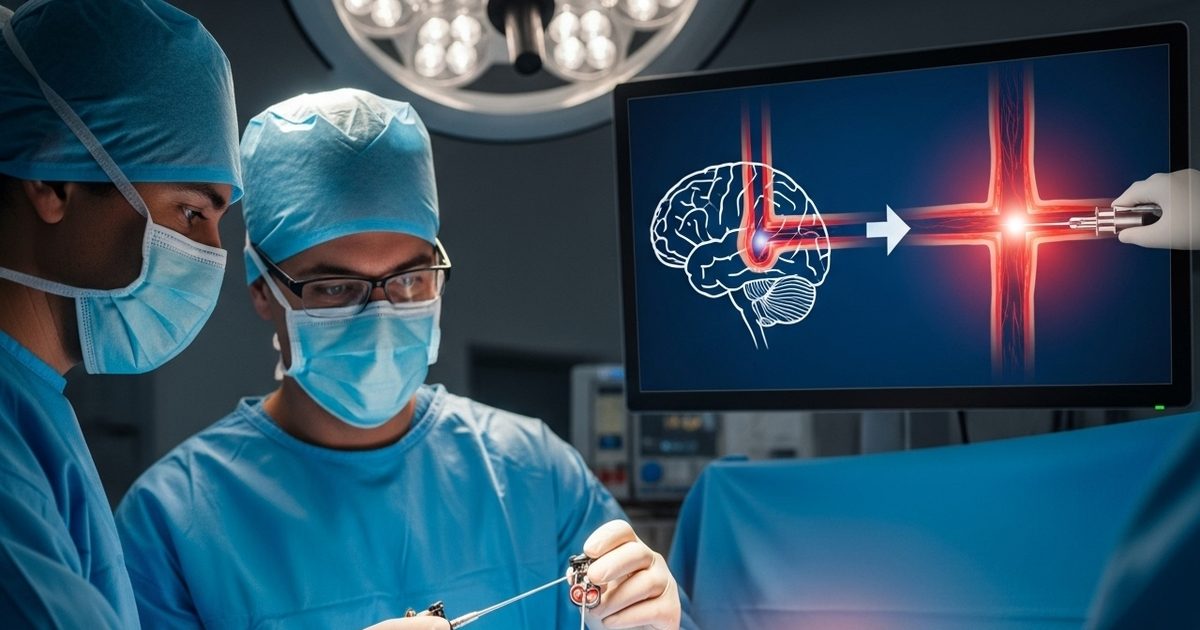
How this special stent works
This device is transported to the vein of the brain through a catheter where the blood clot is trapped. Going there, its net -like structure spreads and captures the entire clot. After this, when the stent is pulled back, the clot also comes out together. In this way, the blood flow in the veins of the brain starts again and the effect of the stroke decreases. In the language of medical science, this technology is considered very advanced and safe.
Before the approval of this device, its multicentric trial was done across the country. It included big hospitals like AIIMS Delhi, Jipmer Pondicherry and CK Birla Hospital-CMRI Kolkata. The special thing is that only CMRI Kolkata took part from Eastern India. After this research, it was proved that this device is also fully effective and safe for Indian population. This is the reason why it has now got regulatory approval.
Kolkata’s CMRI’s Neurology Professor and Trial Investigator Deep Das said that this device will revolutionize India’s stroke care program. He informed that this trial has also proved to be India’s medical research and device validation capability. At the same time, Biman Kanti Rai, who is associated with the Neurosciences Institute of IPGMER, said that the specialty of this device is that it has been designed keeping Indian patients in mind.
The price will be half
Initially, the price of this device is said to be around 3.5 lakh rupees and it can be used only once. But it is a matter of relief that its production will start in India in the next two months. This will reduce its price by 50%. That is, more patients will be able to afford it and the healthcare system will also have a positive effect.
US-based company and Indian Startup Gravity Medical Technology have played an important role in developing this device. Its co-founder and CTO Shashwat Desai said that this device will meet the great need for advanced stroke care in India. At the same time, the trial’s global principal investigator Dilip Yavagal believes that this will make the life saving procedures more economical and accessible now.
Biman Kanti Rai, who is associated with the tele-stroke program of the state health department, said that this device will not only be able to reach advanced stroke treatment in not only metro cities but also in Tier 2 and Tier 3 cities. This will reduce inequality in healthcare and will also help patients from village-towns.
The arrival of Supernova Stent Retriers in India is a big step for the health sector. This will not only save the lives of patients, but it has brought new hope in stroke management. Soon after its production starts in India, it will be even more economical and accessible. It would not be wrong to say that this device is going to give a new height to India’s stroke program in the coming time.
(Disclaimer: The information and information given in this article is based on general recognition. Hindi News18 does not confirm them. Contact the concerned specialist before implementing them.)